|
|





Melatonin receptors are
present on the neurons of the suprachiasmatic nuclei of most
species. This suggests that the secretion of melatonin is
regulated by a negative feedback loop. Experiments in which
the administration of exogenous melatonin affected the circadian
rhythm of locomotor activity in various rodents have also
shown that this hormone can affect the functioning of the
body’s biological clock.
|
|
|
| THE SUPRACHIASMATIC
NUCLEI AND THE PINEAL GLAND |
|
The source of the human body’s circadian
rhythms lies in the suprachiasmatic nuclei (SCNs), which constitute
the central oscillator in the human biological clock. Each of these
two nuclei in the left and right anterior hypothalamus comprises
several tens of thousands of especially small neurons and has its
own spontaneous rhythm of biochemical and electrical activity.
But this rhythm is entrained by and synchronized with daylight
through a neural tract that runs from the retina to the SCNs.
From the SCNs, information
is then relayed to several different brain structures, among
them the pineal gland, via complex multineuronal pathways. Because
the hypothalamus and the pineal gland are located close together
in the diencephalon,
one might expect that the neural pathways connecting them would
be direct, but they are not. Instead, these pathways make a long
detour from the hypothalamus via the spinal cord before returning
to the pineal gland.
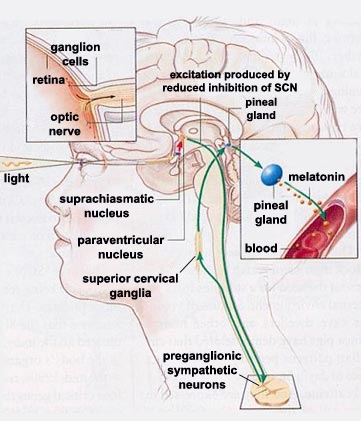 |
During the daytime, the activity of
each SCN reduces that of another part of the hypothalamus,
the paraventricular nucleus (in the diagram
to the left, this inhibition is represented by a red arrow).
The axons of the paraventricular nucleus then descend to
the preganglionic sympathetic neurons of
the lateral horn of the spinal
cord. In turn, these cells modulate the excitability
of the neurons of the superior cervical ganglia, whose
axons finally project to the pineal gland. |
This entire excitatory pathway is shown in
green in the diagram above. Because this circuit contains only
one inhibitory connection—the one from the SCN to the paraventricular
nucleus—one can readily understand how the excitation of
the SCN by daylight ultimately reduces the production of melatonin
by the pineal gland. Conversely, when the sun sets, the effect
of this inhibiting connection diminishes, thus enabling the excitatory
connections to increase the secretion of melatonin by this gland.
| The main neurotransmitter
regulating the activity of the pineal gland is norepinephrine.
When norepinephrine binds to its receptors, it triggers a cascade
of second messengers, including adenylate cyclase and its product,
cyclic AMP. This cyclic AMP contributes to the synthesis of
melatonin from its precursor, tryptophane. |
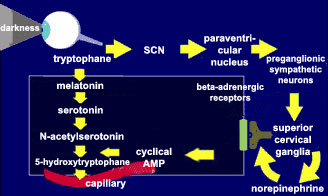 |
This melatonin is released
into the bloodstream, through which it reaches every organ in the
body. That is how it participates in the modulation of the circuits
of the brainstem that ultimately control the
sleep-wake cycle.
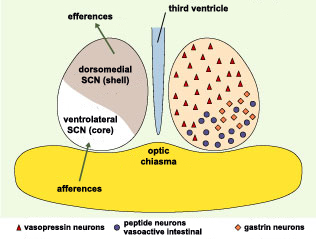
The suprachiasmatic
nuclei were once regarded as homogeneous structures, but
now, like
other nuclei, are instead regarded as sets of distinct,
interconnected functional units. On the basis of the neuropeptides
produced by the various neurons of the SCNs and the functional
organization of the pathways leading into and out of them,
brain anatomists now consider each SCN to consist of a ventral
SCN and a dorsal SCN.
The neurons of the ventral SCN are
now believed to function not so much as clocks but rather
as the location in the SCN that receives and responds to
external inputs, while the neurons of the dorsal SCN are
believed to constitute the SCN’s actual robust, endogenous
clock. This view is supported by certain jet-lag experiments
which have shown that in rats, the process by which a light
stimulus resets the internal clock occurs far more rapidly
in the ventral SCN than in the dorsal SCN.
Scientists have now discovered that
the neurotransmitter GABA excites the cells of the dorsal
SCN but inhibits those of the ventral SCN. These opposing
effects might influence the differing reaction times of these
two sub-regions when someone travels across several time
zones. This discovery thus opens new insights into the mechanisms
behind the disturbing symptoms of jet
lag.
Source: Hugues
Dardente and Nicolas Cermakian, Médecines/Science,
Volume 21, Number 1 (January 2005)
|
|
|







