|
|

 |
 |
 |

 |
 |
In addition to benzodiazepines, antidepressants,
and neuroleptics, certain antihistamines (such as hydroxyzine)
have sedative properties and are used to treat certain
relatively short-term forms of anxiety.
Some serotonergic
5-HT1A receptor agonists, such as buspirone, by activating
the serotonergic autoreceptors, reduce the secretion of serotonin,
thus producing an anxiolytic effect comparable to that of medications
which activate GABA receptors, such as benzodiazepines.
The fact that certain serotonin-reuptake-inhibiting antidepressants
(substances such as fluvoxamine, which increase the secretion
of serotonin) can be used to treat obsessive-compulsive disorder
shows at least two things. First, the amount of a neurotransmitter
is too vague a criterion to account on its own for all of the
complex phenomena involved in anxiety disorders. Second, anxiety
and depression probably
involve some similar biological mechanisms. For example, we
know that people who have anxiety disorders in childhood are
at greater risk for developing depression later in life.
|
|
|
 |
|
Tranquilizers should not be prescribed automatically whenever
anxiety symptoms are present, because these symptoms are
not considered pathological unless they become disabling
for the person experiencing them. At that point, they warrant
specific treatment, most often with benzodiazepines, which
can be prescribed temporarily without any significant negative
health effects. In such cases, benzodiazepines can generally
effectively reduce the anxiety that the person is experiencing,
but they cannot attack its underlying causes. |
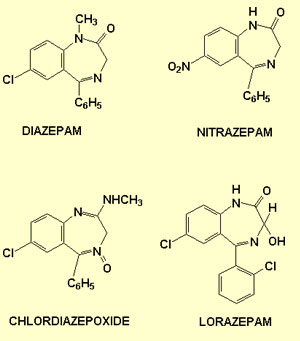
The term “benzodiazepine” refers
to the general class of chemicals to which these molecules belong.
Each member of this class has a generic name or is simply known
by its trade name. The following table lists some of the most common
benzodiazepines.
| Generic name (active ingredient) |
|
Marketed under the trade name(s): |
| |
|
|
| Diazepam |
|
Valium, Vivol, T-Quil, Valrelease |
| Lorazepam |
|
Ativan, Alzapam, Loraz |
| Alprazolam |
|
Xanax, Alprazolam Intensol |
| Chlordiazepoxide |
|
Librium, Novopoxide, Libritabs |
| Flurazepam |
|
Dalmane, Novoflupam, Somnol |
| Nitrazepam |
|
Mogadon |
| Triazolam |
|
Halcion |
| Temazepam |
|
Restoril |
| Oxazepam |
|
Serax |
Researchers have long known that benzodiazepines bind
to GABA-A
receptors, thus facilitating the opening of their chloride
channels and potentiating GABA's inhibitory effect. But now researchers
are increasingly finding that the
specific effects of each type of benzodiazepine seem to be
directly linked to the various types of GABA-A receptors to which
it can bind (GABA-A receptors are made up of sub-units designated
alpha, beta, and gamma, and the various types of GABA-A receptors
represent varying arrangements of these sub-units).
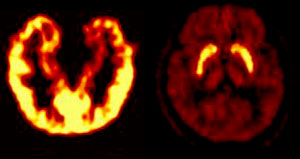
Left: distribution of benzodiazepine
receptors in the brain. Right: distribution of D2 dopaminergic
receptors
Source: CERMEP, Hôpital Neuro-Cardiologique de Lyon |
|
Research suggests that
different benzodiazepines may have differing affinities for
these various types of GABA-A receptors, which may in turn
explain why one benzodiazepine has more of an anxiolytic effect
while another has more of a sedative effect. Also, some studies
show a marked variation in the distribution of these various
kinds of benzodiazepine receptors within the brain. For example,
the type of benzodiazepine receptor that predominates in the
motor and sensory cortexes is not the same one that predominates
in the circuits
of the amygdala and the other structures of the limbic
system. |
These studies thus support the idea that
the distinctive effects of various benzodiazepines are associated
with the different types of receptors and their distribution in
the brain.
Some studies
seem to show that endocannabinoids—substances that
are analogous to the active ingredient in cannabis but that
are produced inside the human body—help to extinguish
painful memories through their inhibitory effect
on the internal
circuits of the amygdala.
In one study, for example, mice that had been genetically altered
so that they did not express the endocannabinoid receptor had
much more trouble in extinguishing a conditioned fear, even
though they did not display any other memory problems. (The
same result was also obtained when the endocannabinoid receptor
was blocked with an antagonist.)
While the mice were being exposed to the stimulus that led
to the extinction of the response, the researchers also found
high levels of endocannabinoids in the basolateral
nucleus of the amygdala, which is known to be involved
in the extinction of painful memories. This extinction seems
to involve the activation of NMDA
receptors by glutamatergic neurons. Endocannabinoid
receptors are known to have an inhibitory effect on the GABAergic
inhibitory neurons in the basolateral nucleus. Hence one
can see how inhibition of these inhibitory neurons by endocannabinoids
might lead to activation of the NMDA receptors and thus facilitate
extinction of the conditioned fear.
The psychotherapeutic treatment for many anxiety disorders
(including phobias)
is based on a process of extinction (placing the patient in
contact with the feared object in the safe setting of the therapist's
office). Hence, if a substance that increased the levels of
endocannabinoids at the right places in the patient's brain
could be administered during psychotherapy, it might facilitate
a cure. (This probably would not be the case if the patient
simply smoked a joint, flooding the entire brain with THC.)
Lastly, there are other molecules that also may help to extinguish
painful memories. Some of these molecules activate the NMDA
receptors in the amygdala, which are also involved in the
process of extinction.
|
|
|