|
|
| Funding for this site is provided by readers like you. | |
|
|
|
|
|||||
|
|
|||||||
|
|
|
|
|
|
|
|
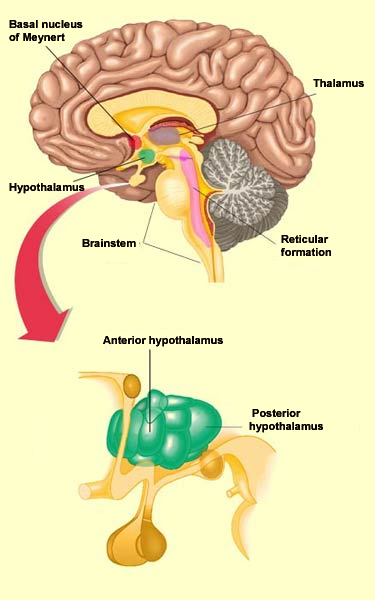
The regulation of wakefulness is essential for survival and involves several different redundant structures in the brain. None of these structures, taken in isolation, is indispensable for activation of the cortex. But three of these brain structures that send projections to the cortex are sufficient to maintain the desynchronized EEG pattern that is characteristic of wakefulness. These structures are the posterior hypothalamus, the basal telencephalon, and the intralaminar nuclei of the thalamus. Together they are often referred to as the “executive network”. Stimulating the posterior hypothalamus produces a state of wakefulness comparable to that induced by stimulating the reticular formation in the brainstem. The activity of the posterior hypothalamus diminishes naturally during sleep, when it releases less histamine, a molecule that it uses as a neurotransmitter. The antihistamines that people take for allergy symptoms are known to cause some sleepiness, by reducing the activity of histamine.
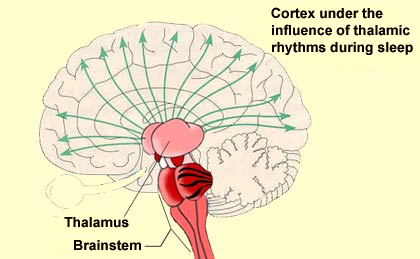
In the situations just described, the cortical activation that causes wakefulness results from the direct stimulation of the cortical neurons by the various components of the wakefulness network. But these cortical neurons can also be activated in another way: by the inhibition of those neurons that naturally inhibit cortical activity. And that is exactly what the GABAergic neurons located in the posterior hypothalamus and the basal telencephalon do: they inhibit other, cortical GABAergic neurons. This executive network for wakefulness is itself activated by other systems arising in the brainstem. It is thus all of these wakefulness signals that stop reaching the cortex at the onset of non-REM sleep. They are interrupted at the thalamus, which serves as a true gatekeeper to the cortex and is greatly influenced by the diffuse neuromodulatory systems of the brainstem.
More specifically, it is the rhythmic activity pattern established by the thalamocortical neurons of the intralaminar nuclei that disconnects the cortex from internal and external signals at the onset of non-REM sleep. In contrast, during the REM phases of sleep, the thalamus probably continues to pass at least some of these signals on to the cortex, at least in some fragmentary, filtered, or distorted form. Lastly, we cannot overlook the fundamental role that the anterior
hypothalamus plays
in the process of falling asleep. This structure, and in particular
its preoptic area, appears to be sensitive to the serotonin
released during waking periods. Apparently, when serotonin
stimulates this preoptic area of the anterior hypothalamus, its
GABAergic neurons in turn inhibit the posterior hypothalamus, thus
encouraging sleep. Damage to these
GABAergic neurons is known to cause insomnia, whereas stimulating
them causes experimental subjects to fall asleep rapidly. |
|
||||||||||||||||||||||||||||||||||||||||
|
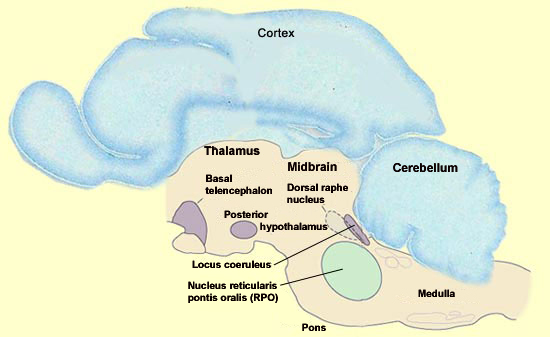
The amygdala is one of the parts of the brain that is most active during REM sleep, but this state is actually generated deep in the brainstem. However, the set of cortical and limbic structures involved in REM sleep do not just passively submit to orders issued by the brainstem. On the contrary, the particular kind of dream images associated with REM sleep are the result of a dynamic interaction between certain key structures in the brainstem and the rest of the brain. The discovery that REM sleep occurs in almost all mammals opened the door to the first animal experiments with REM sleep, which were conducted by French neurobiologist Michel Jouvet in the early 1960s. By studying the effects of lesions and electrical stimuli in the brain, particularly in cats, Jouvet was gradually able to home in on the brainstem as the location of the neurons that trigger REM sleep. One of Jouvet’s methods involved dividing the brain into two halves and trying to see which of them maintained the function in question. He then divided the “winning”half and repeated this process to further narrow down the brain area involved in this function, and so on until he had isolated certain essential nuclei. Because each part of the brain is usually densely interconnected with the others, one might expect that the slightest subdividing of the brain would eliminate the electrical activity specific to dreaming. But surprisingly, the parts of the brainstem that generate REM sleep can continue to do so even if they are disconnected from practically all of the rest of the brain. In contrast, the destruction of even a small portion of the brainstem itself can prevent the expression of REM sleep. What then are these nuclei that Jouvet found in a very small portion of the brainstem and that are both necessary and sufficient for the neurological activity of REM sleep? They are located essentially in the pons, and in particular in its rostral portion, where it meets the midbrain. One of these nuclei that are essential for REM sleep is the nucleus reticularis pontis oralis (RPO), which extends from the caudal midbrain to the rostral pons. A population of neurons in this area is selectively active during REM sleep. Lesion studies of this small area of the pontine tegmentum have also shown that it is required in order for normal REM sleep to occur.
REM sleep can be induced by the microinjection of acetylcholine agonists into this small area. It is not known whether this area is sufficient to generate all of the aspects of REM sleep, for example, the way the suprachiasmatic nucleus suffices to maintain all aspects of the circadian cycle. That a sudden elevation in the activity of the cholinergic neurons of the pons is necessary for the onset of REM sleep may seem somewhat strange, because the brain’s acetylcholine systems are known for their role in wakefulness.How then can this system associated with wakefulness be activated during one of the phases of sleep? The answer probably lies in the simultaneous reduction in the activity of two other nuclei that produce other wakefulness neurotransmitters, a reduction that is just as necessary for REM sleep as the increase in cholinergic activity. The two nuclei in question are the dorsal raphe nucleus, a group of serotonergic neurons, and the locus coeruleus, a group of noradrenergic neurons, and both of them are located in the rostral portion of the pons. REM sleep cannot begin unless all activity in these two main
aminergic systems of the brainstem ceases. The serotonergic and
noradrenergic neurons in these systems are referred to as REM-off neurons
because of their inactivity during REM sleep, and they can be
said to act as a sort of permissive system for REM sleep, in
the sense that REM sleep becomes possible only when their activity
has ceased. The shutdown of these neurons might, for example,
help to suppress consciousness during REM sleep. Also, the periods
of REM sleep end when these aminergic neurons become active again. |
| |
|
|
|
|
|
|
|
|